Dilution is a procedure by which the concentration of a solution is lowered, usually by adding a diluent. However, dilution can also occur by a system that removes solute from the solution. Though it sounds strange, it is a routine exercise in the kitchen when a potato is added to a very salty food to remove extra salt. The nutrition will taste less salty as the potatoes engross their salt content.
Table of Contents
What Does it Contain?

To exemplify the concept of dilution, the unknown is better than imagining a startup’s property as a cake. When the time arises, the needs of the plan need more financing, and the possibility of making a capital increase is careful: the size of the cake increase so that others can get a share (in exchange for their consistent investment).
When this expansion of the size of the cake occurs, two scenarios can occur. First, those nominees who already have a part of the possessions can choose to buy a certain amount of the new shares with the intention that their percentage does not reduce due to the entry of new investors. On the other hand, if he declines this possibility. The old investor will see that the percentage representing his share is now smaller as the size of the pie increases.
Thus, the dilution can occur in two ways because some of the old associates make a new contribution and the proportion of those who do not contribute the money increase. Because new shareholders enter the business. In any case, the cause and the main consequence agree: the startup needs funding, and to obtain it, those involved with the project give up part of their property.
The Benefits of Dilution
In short, dilution should be more desired than feared by investors. On the one hand, the very nature of a company makes this the logical and obvious step for the startup to grow and reach new goals.
But, if the new investment manages appropriately, its main consequence will be the increase in the startup’s valuation. Ultimately affecting the growth in value of the investor’s shares.
What are Solutions and Dilutions?
In chemistry, solutions and dissolutions represent a homogeneous mixture of two or more substances. When combined or linked, are modified, creating new meaning with the properties of each one. The new substance with its characteristics, stored in a refrigerant tube, receives the solution’s name, or dissolution, depending on where you read it and who tells you.
Solutions and solutions are present in practically all combinations of substances, for example, seawater. So you may think that it only represents water. Still, in reality, it is a solution of different types of salts and regular water, which gives it that characteristic salty ‘taste.’
As you may have noticed, two elements are needed to make a solution. That is solute and solvent, which, when mixed, react with each other, bringing the components together and varying in intensity depending on the amount they combine.
What is the Difference Between Solutions and Dilution?
In terms of chemistry, there is no difference between solutions and solutions since they are the same thing. Therefore, it calls for a solution, but the word ‘dissolution’ is the most correct linguistically speaking in recent years.
On the one hand, we have the word ‘solutions,’ which is a direct translation of the word ‘solutions’ in English, which is the action of mixing. And on the other, the term ‘dissolution’ is the act of dissolving two substances to create a new one.
Therefore, you will refer to the same concept without changing anything essential when using the word dissolution or solution. Of course, some books prefer to take one action over the other, but it’s still the same.
What Does it Mean to Perform a Dilution?
In chemistry, dilution is the reduction in the concentration of a chemical in a solution. Dilution consists of lowering the amount of solute per unit volume of solution.
In any case, how do you do a dilution?
Dilution consists of lowering the amount of solute per unit volume of solution. It achieves by adding more diluent to the same amount of solute. A small portion of an aliquot solution takes and then introduced into more solvent—the initial volume of solution.
In the following way, what does dilution 1 1000 mean?
Sometimes it is necessary to make dilutions: 1: 1000 = 1 mg per ml -> Ampoule or syringe with 1 mg in 1 ml.
Another question would be, what is the difference between dissolution and dilution?
A typical mistake is to confuse ” dissolution ” with ” dilution. Two different processes: a dissolution is to prepare a solution by having the solute and solvent initially separated. A dilution is just adding more solvent to the already prepared solution.
What is 1 100 Dilution?
For example, taking a milliliter (mL) of H<sub>2</sub>SO<sub>4</sub> (sulfuric acid) with a concentration of 1 molar (one mole of a substance in one liter of solution ), and dissolve it in 99 ml of distilled water. A dilution of 1/100 ( one in one hundred ) is complete. The resulting final concentration will be 0.01 molar.
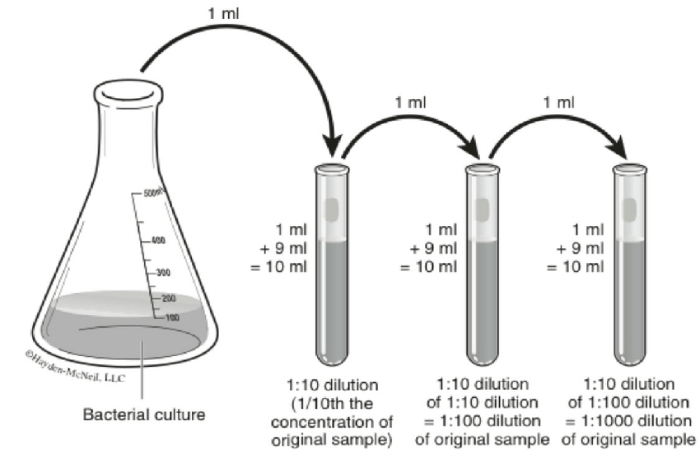
What is a 1 to 10 Dilution?
When 1 mL of a bacterial suspension is mixed with 9 mL of saline, making a total of 10 mL, it represents a 1:10 dilution ( also expressed as 1/10 or 10 – 1 ). The dilution factor is the converse of the dilution; inverse means that you reverse the two numbers in the fraction.
Conclusion
Dilutions can play an essential role in treating an unknown substance. For example, you can perform a dilution to reduce the concentration of the analyte measure so that it is within range and still. It can also help you eliminate interference from other substances present in the sample, which can alter the model—artificially analysis.
An analyte is a compound you want to test for in the sample. So, for example, if a chlorine test is performes, chlorine would be the analyte.

